- ISO – Medical devices – A practical guide has been authored by technical experts of ISO/TC 210. The handbook is intended to guide organizations in the development, implementation and maintenance of their quality management system in accordance with ISO 13485.
- Download this ISO 13485 Documentation Template for free today. This QUALITY MANUAL Document Template is part of the ISO 13485 Documentation Toolkit. The toolkit combines documentation templates and checklists that demonstrate how to implement this standard through a step-by-step process. In addition, you can access help from our experts to keep.
- The revised ISO 13485 was published on 1 March 2016. IAF Resolution 2015-13 details a transition period of three years from the date of publication. Certification bodies have to apply to transition its accreditation. Once approved, CBs can issue certificates to ISO. In the interim, CBs are able to conduct audits, provided auditors are.
Is a stand-alone standard, it is structured similar to ISO 9001:2008, which has been superseded by ISO 9001:2015. For the convenience of users, Annex B of the standard shows the. View the 'EN ISO /AC:2016' standard description, purpose. Or download the PDF of the directive or of the official journal for free This website uses cookies to ensure you get the best experience on our website.
- 26.10.2019
ISO Free Downloads
 The new edition of the ISO standard was published on March 1 , concluding almost five years of intense discussion and development by experts around the world to improve and update the standard with new European requirements and other international regulatory changes, implemented since its previous revision in Organizations complying with this standard, such as medical devices manufacturers, as well as suppliers and external parties that provide products or services to manufacturers, will be able to demonstrate compliance with regulatory requirements, manage risk, ensure best practice for quality and safety, improve processes and provide confidence to patients and users. The new ISO focuses on how companies should manage risk-based decisions related to purchasing, design, development, manufacturing, production control activities and other aspects of the quality management system. Some of the key changes between the and version include:. SGS will soon provide materials, services and courses to help make the transition in the best possible conditions. SGS is currently making arrangements with the appropriate accreditation authorities to confirm the plan to start the certification service for ISO and will soon communicate accordingly. Click here for more information on ISO
The new edition of the ISO standard was published on March 1 , concluding almost five years of intense discussion and development by experts around the world to improve and update the standard with new European requirements and other international regulatory changes, implemented since its previous revision in Organizations complying with this standard, such as medical devices manufacturers, as well as suppliers and external parties that provide products or services to manufacturers, will be able to demonstrate compliance with regulatory requirements, manage risk, ensure best practice for quality and safety, improve processes and provide confidence to patients and users. The new ISO focuses on how companies should manage risk-based decisions related to purchasing, design, development, manufacturing, production control activities and other aspects of the quality management system. Some of the key changes between the and version include:. SGS will soon provide materials, services and courses to help make the transition in the best possible conditions. SGS is currently making arrangements with the appropriate accreditation authorities to confirm the plan to start the certification service for ISO and will soon communicate accordingly. Click here for more information on ISO How to Simplify Your Compliance with the New ISO 13485:2016
The publication and release of ISO earlier this year is a significant movement for the medical device industry. The last major revision of this quality management system standard happened back in And what does this mean for your quest to have a quality management system to meet all of the major global medical device quality system regulatory requirements?
Association for the Advancement of Medical Instrumentation
Everything you need to know about what makes MasterControl the right solution for you. Take a look at our pricing plans and request an estimate that works for your business. Want to see what MasterControl has done for great organizations? Explore our many case studies. We are growing fast and looking for innovative and creative people to join the team. Learn about the variety of partnerships and strategic benefits available in our partner network.
2. ISO 13485:2016 CLAUSE 4.2 DOCUMENTATION REQUIREMENTS
ISO is an international quality management standard for medical devices. Systemic requirements. Management requirements.
See our product tour or contact our main ISO expert who is here to assist you in your implementation. No matter if you are new or experienced in the field, this book gives you everything you will ever need to learn more about certification audits. In this book Dejan Kosutic, an author and experienced ISO consultant, is giving away his practical know-how on managing documentation. No matter if you are new or experienced in the field, this book gives you everything you will ever need to learn on how to handle ISO documents. No matter if you are new or experienced in the field, this book gives you everything you will ever need to learn about preparations for ISO implementation projects. No matter if you are new or experienced in the field, this book gives you everything you will ever need to learn and more about internal audits. Understanding ISO can be difficult, so we have put together this straightforward, yet detailed explanation of ISO
Type the code from the image. Store Home. You must enter a search term before you press the Search button. Place Order. Your cart is empty. Product Categories.
.
Iso 13485 2016 Free Copy
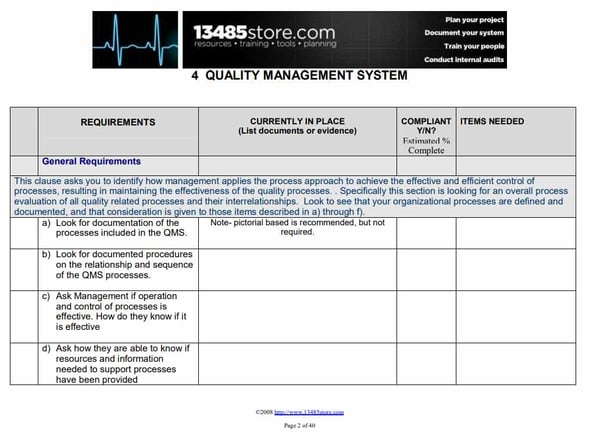
Iso 13485 Download
